Eventually, these individual laws were combined into a single equation—the ideal gas law—that relates gas quantities for gases and is quite accurate for low pressures and moderate temperatures. We will consider the key developments in individual relationships , then put them together in the ideal gas law. The behavior of gases can be described by several laws based on experimental observations of their properties. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (Amontons's law). The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (Charles's law). The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant (Boyle's law).
Under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules (Avogadro's law). The noble gases xenon and helium are known to possess neuroprotective properties. Xe is considered the golden standard neuroprotective gas. In contrast, He has a lower molecular weight and higher thermal conductivity and specific heat than those of nitrogen, but is unfortunately far less potent than Xe at providing neuroprotection. Therefore, combining Xe with He could allow obtaining, depending on the gas inhalation temperature and composition, gas mixtures with neutral or hypothermic properties, the latter being advantageous in term of neuroprotection. However, calculating the thermal properties of a mixture, whatever the substances – gases, metals, rubbers, etc. – is not trivial.
First, we must determine the question, which is to calculate the volume of a quantity of gas at a given temperature and pressure. In a second step, after establishing a basis, we must convert the mass of methane that will be the basis into pound moles. Third, we must convert temperature in degrees Fahrenheit into absolute degrees Rankin and, fourth, convert pressure from psig into psia.
Fifth, we must select the appropriate ideal gas constant and use it with a rewritten form of Equation 4.11 to determine the volume of 11.0 lbs of methane gas. Finally, we can substitute the values previously determined into the rewritten equation to calculate the volume. This relationship between temperature and pressure is observed for any sample of gas confined to a constant volume. An example of experimental pressure-temperature data is shown for a sample of air under these conditions in .
How Do You Find The Final Volume Of A Gas An example of experimental pressure-temperature data is shown for a sample of air under these conditions in Figure 9.11. An ideal gas is a hypothetical construct that may be used along with kinetic molecular theory to effectively explain the gas laws as will be described in a later module of this chapter. Although all the calculations presented in this module assume ideal behavior, this assumption is only reasonable for gases under conditions of relatively low pressure and high temperature. In the final module of this chapter, a modified gas law will be introduced that accounts for the non-ideal behavior observed for many gases at relatively high pressures and low temperatures.
An example of experimental pressure-temperature data is shown for a sample of air under these conditions in Figure 3. Like the other ideal gas laws, Avogadro's law only approximates the behavior of real gases. Under conditions of high temperature or pressure, the law is inaccurate. The relation works best for gases held at low pressure and ordinary temperatures. Also, smaller gas particles—helium, hydrogen, and nitrogen—yield better results than larger molecules, which are more likely to interact with each other. We have just seen that the volume of a specified amount of a gas at constant pressure is proportional to the absolute temperature.
In addition, we saw that the volume of a specified amount gas at a constant temperature is also inversely proportional to its pressure. We can correctly assume that pressure of a specified amount of gas at a constant volume is proportional to its absolute temperature. Let us also add the fact that the volume at constant pressure and temperature is also proportional to the amount of gas. Similarly, the pressure at constant volume and temperature is proportional to the amount of gas. Thus, these laws and relationships can be combined to give Equation 4.10. Compressing a gas initiates changes in its characteristics.
Because you're compressing it, the volume of space the gas occupies decreases, but a lot more happens than this alone. Compression changes the temperature and pressure of the gas too, depending on the specifics of the situation. You can understand the changes that occur using an important law in physics called the ideal gas law. This law simplifies the real-life process somewhat, but it is useful in a wide range of situations.
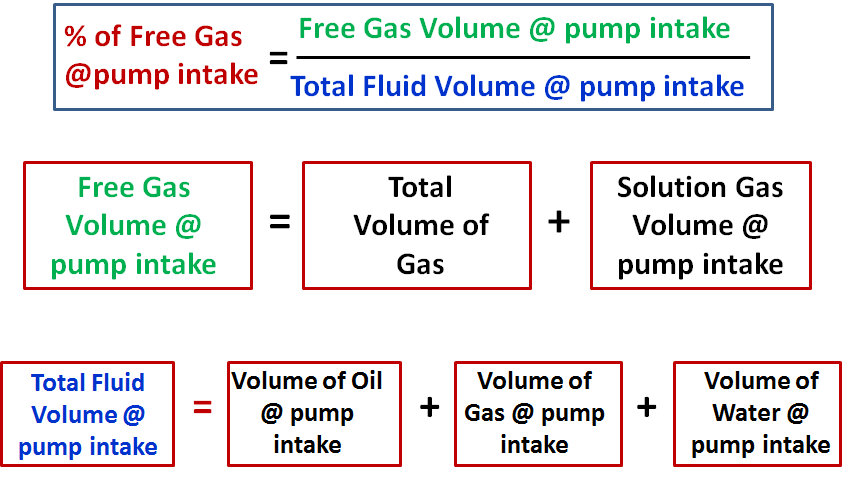
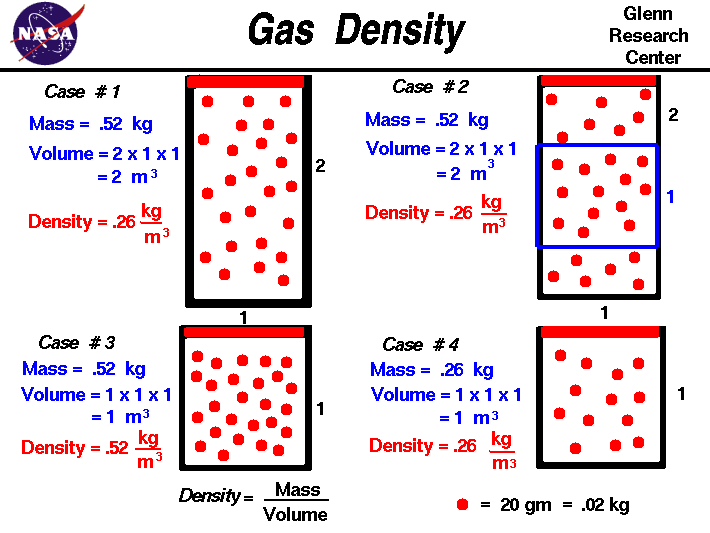
The stateof agasis defined by various propertieswhich we can observe with our senses, including the gaspressure ,temperature , mass (number of moles - m), andvolume which contains the gas. It is observed that, if we have a certain amount of gas present, the value of the temperature and pressure does not depend on the amount of gas which we examine. If we insert a plate into the tank which cuts the volume in half, the temperature in each half remains the same, as does the pressure.
The value of pressure and temperature does not depend on the amount of gas used in the measurement. The mass of the gas, on the other hand, does depend on the volume. The mass in each section of the tank is one half the mass of the entire tank. The mass depends on the volume and, in turn, the volume depends on the mass. If we maintain the pressure and temperature of this gas and fill an object which can vary its volume, like a balloon, or a cylinder with a sliding end, the final volume depends directly on the amount of the gas that we inject. Notice that if we hold the volume constant and inject mass, the value of pressure and temperature change, but in the example on this slide, the total mass is kept constant.
The attraction or repulsion between the individual gas molecules and the container are negligible. Further, for an ideal gas, the molecules are considered to be perfectly elastic and there is no internal energy loss resulting from collision between the molecules. Such ideal gases are said to obey several classical equations such as the Boyle's law, Charles's law and the ideal gas equation or the perfect gas equation. We will first discuss the behavior of ideal gases and then follow it up with the behavior of real gases.
It is possible to calculate the final volume or pressure of an ideal gas using the various gas laws such as Boyle's law. According to this particular law, the pressure is inversely proportional to the volume. The necessary condition is that temperature should be constant.
In addition, in line with its scarcity, Xe suffers an excessive cost of production that is a major obstacle to its clinical development. Avogadro's gas law states the volume of a gas is proportional to the number of moles of gas present when the temperature and pressure are held constant. This example problem demonstrates how to use Avogadro's law to determine the volume of a gas when more gas is added to the system.
In an isenthalpic process, system enthalpy is constant. In the case of free expansion for an ideal gas, there are no molecular interactions, and the temperature remains constant. For real gasses, the molecules do interact via attraction or repulsion depending on temperature and pressure, and heating or cooling does occur. For reference, the Joule–Thomson coefficient μJT for air at room temperature and sea level is 0.22 °C/bar. Depending on the units of measure for the pressure, the volume, the number of moles, and the absolute temperature, some values for the ideal gas constant R are given in Table 4.1 and Appendix C for different units-of-measure systems. If we partially fill an airtight syringe with air, the syringe contains a specific amount of air at constant temperature, say 25 °C.
If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; if we pull out the plunger, the volume increases and the pressure decreases. This example of the effect of volume on the pressure of a given amount of a confined gas is true in general. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. In fact, if the volume increases by a certain factor, the pressure decreases by the same factor, and vice versa. Volume-pressure data for an air sample at room temperature are graphed in . Volume-pressure data for an air sample at room temperature are graphed in Figure 9.13.
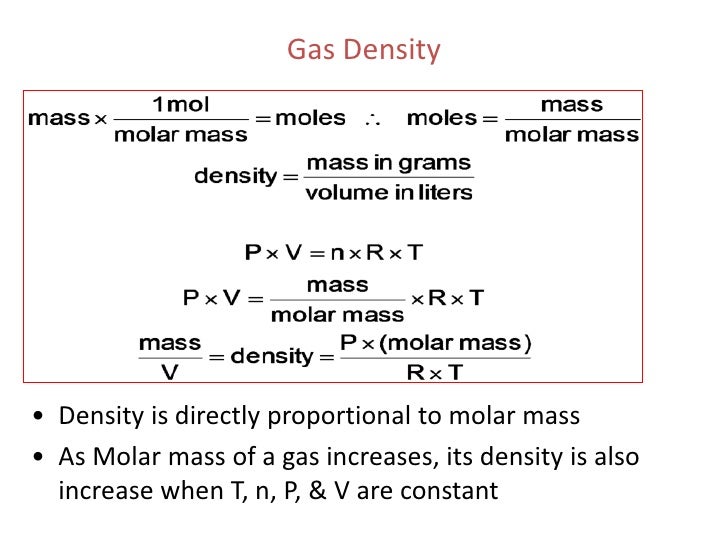
For a constant volume and amount of air, the pressure and temperature are directly proportional, provided the temperature is in kelvin. The ideal gas equation contains five terms, the gas constant R and the variable properties P, V, n, and T. Specifying any four of these terms will permit use of the ideal gas law to calculate the fifth term as demonstrated in the following example exercises. Volume-pressure data for an air sample at room temperature are graphed in Figure 5.
As a result, the highest is the volume proportion of He (Ya-Yc), the lowest is the volume proportion of Xe (Yc-Yb) in the gas mixture. However, whether body temperature as measured in rats after breathing Xe-He gas mixture would be consistent with the predictive results shown in Table 1 remains to be investigated. Also, translation to humans could need establishing new regression lines regarding the effects of Xe-O2 and He-O2 gas mixtures on body temperature. The volume and temperature are linearly related for 1 mole of methane gas at a constant pressure of 1 atm. If the temperature is in kelvin, volume and temperature are directly proportional.
The line stops at 111 K because methane liquefies at this temperature; when extrapolated, it intersects the graph's origin, representing a temperature of absolute zero. Temperature is sometimes measured with a gas thermometer by observing the change in the volume of the gas as the temperature changes at constant pressure. The hydrogen in a particular hydrogen gas thermometer has a volume of 150.0 cm3 when immersed in a mixture of ice and water (0.00 °C).
When immersed in boiling liquid ammonia, the volume of the hydrogen, at the same pressure, is 131.7 cm3. Find the temperature of boiling ammonia on the kelvin and Celsius scales. In another lesson, you learned about ideal gases and the ideal gas equation. But since real gases behave similarly to ideal gases at normal temperatures and pressures, we can use the ideal gas equation to predict the behavior of real gases under these conditions.
For a gas, which variables are inversely proportional to each other if all other conditions are held constant? Where the heat capacity ratio (CP/CV) is 5/3 for a monatomic gas such as helium. Since times and lengths can be measured very accurately, the measurement of acoustic velocities by the detection of successive resonances in a cylindrical cavity appears to offer an ideal way to measure temperature. This is not completely correct, however, since boundary effects that affect the velocity of sound are important even for the simplest case in which only one mode is present in the cavity .
These effects unfortunately become larger as the pressure is reduced. An excellent theory relates the attenuation in the gas to these velocity changes, but the situation is very complex and satisfactory results are possible only with complete attention to detail. An alternative configuration uses a spherical resonator in which the acoustic motion of the gas is perpendicular to the wall, thus eliminating viscosity boundary layer effects. The most reliable recent determination of the gas constant, R, is based on very careful sound velocity measurements in argon as a function of pressure at 273.16K, using a spherical resonator.
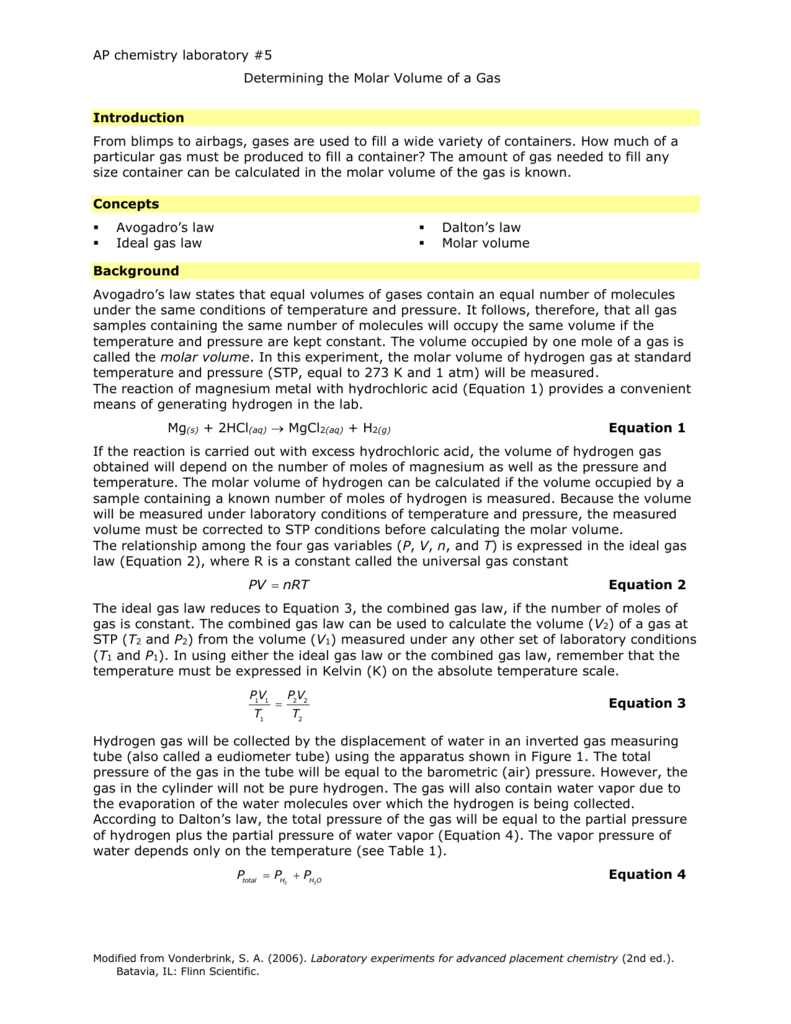
A certain mass of gas has a volume of 1000ft3 at 60psig. If temperature is constant and the pressure increases to 120psig, what is the final volume of the gas? Kinetic particle reasoning - increasing the temperature increases the kinetic energy of the molecules giving more forceful collisions which push out the gas at constant pressure. In the past lots of measurements have been made to investigate how the pressure and volume of a given mass of vary at constant temperature and how the pressure and volume gas of a fixed mass of gas varies with temperature . This resulted in the formulation of the laws of gases described in the next section 4a. Along with how to use them in calculations and problem solving.
Under these conditions, the volume of the gas will vary inversely with the absolute pressure. This equation calculates a pressure given the corresponding elements of the equivalence; Initial pressure, Initial volume, and temperature. To calculate the final temperature T2 of the gas, use the ideal gas law. If you compress a fixed number of moles of gas, and you do this in an isothermic process , the pressure must increase to account for the smaller volume on the left of the equation. Similarly, when you cool a gas at a fixed pressure, its volume decreases – it compresses.
Let Cv and Cp denote the molar heat capacities of an ideal gas at constant volume and constant pressure respectively. The preceding example was actually a simple problem that has been made more complicated here. The simple solution can provide an estimate for Step 13 of the problem-solving technique in Chapter 1, "Introductory Concepts," in which we judge our results. First, the cracking of ethane into ethylene with the hydrogen being given off doubles the number of moles of gas.
Second, the increase of temperature, from 810°R (350°F) to 2,010°R (1,550°F), also more than doubles the volume. Thus, the volume of the gases would be about quadrupled, which approximates and confirms the 4.7 times increase in our previous calculation. Imagine filling a rigid container attached to a pressure gauge with gas and then sealing the container so that no gas may escape. If the container is cooled, the gas inside likewise gets colder and its pressure is observed to decrease.
Since the container is rigid and tightly sealed, both the volume and number of moles of gas remain constant. If we heat the sphere, the gas inside gets hotter () and the pressure increases. The procedure for constant-volume gas thermometry is very much the same as that for isotherm thermometry, but detailed bulb pressure data are taken as a function of temperature for one "filling" of the bulb at the standard temperature. To first order, pressure ratios are equal to temperature ratios, with thermodynamic temperatures calculated using known virial coefficients. If the constant-volume gas thermometer is to be used in an interpolating gas thermometer mode (as for the ITS-90), the major corrections are due to the nonideality of the gas.
Therefore, according to Charles' law for an ideal gas at constant pressure, the volume will change in the same proportion as its temperature. Thus, a 20% increase in temperature will cause a 20% increase in volume as long as the pressure does not change. Similarly, if volume is kept constant, a 20% increase in temperature will result in the same percentage in increase in gas pressure. In order to study this problem, much research work has been done. The result of this research work is a term called the compressibility factor.
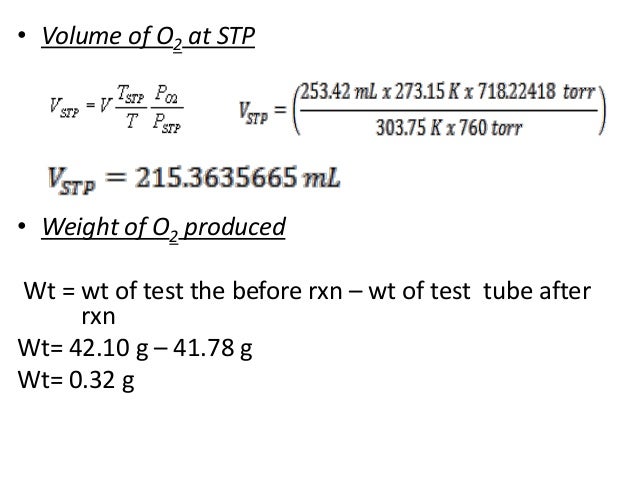
This is also sometimes called the supercompressibility factor. The American Gas Association has sponsored research that defines these factors for all of the conditions to which natural gas is normally exposed. The factors have been found to be affected by temperature, pressure, gas specific gravity, and gas composition, particularly the inert content of the gas. This information is in the form of tables of values as well as the equations for calculating the factor values from the gas properties and conditions. There are a number of chemical reactions that require ammonia.
In order to carry out the reaction efficiently, we need to know how much ammonia we have for stoichiometric purposes. Using gas laws, we can determine the number of moles present in the tank if we know the volume, temperature, and pressure of the system. If we heat the sphere, the gas inside gets hotter (Figure 9.10) and the pressure increases. If we heat the sphere, the gas inside gets hotter and the pressure increases. Charles's law is an experimental gas law that describes how gases tend to expand when heated. When the pressure on a sample of a dry gas is held constant, the Kelvin temperature and the volume will be in direct proportion.
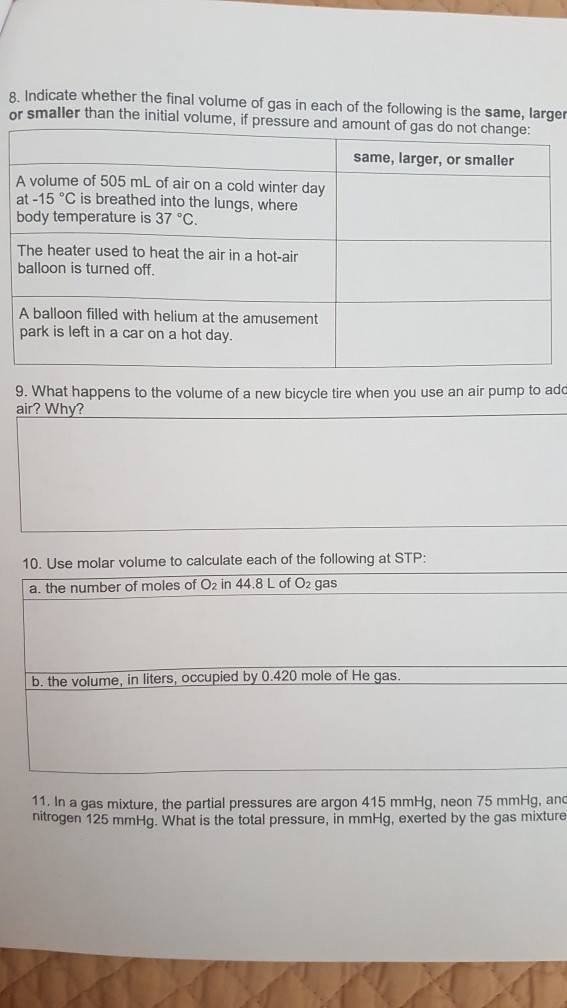
This law describes how a gas expands as the temperature increases; conversely, a decrease in temperature will lead to a decrease in volume. A gas has an initial volume of 2.4 L at a pressure of 1.5 atm and a temperature of 273 K. The pressure of the gas increases to 4.5 atm, and the temperature of the gas What is the final volume of the gas? 0.92 L 6.3 L 8.3 L Subrit Save and Exit Mark this and teturn ContentViewers/AssessmentViewer/Activit. The Peng-Robinson equation of state was used to calculate the volume of 100% methane gas as a function of pressure and temperature . This equation expresses fluid properties in terms of the critical properties and acentric factor of each species involved.





















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.